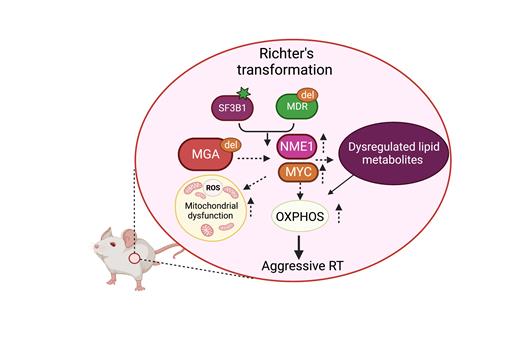
Despite advances in targeted therapies for chronic lymphocytic leukemia (CLL), Richter's transformation (RT) remains a clinical challenge. RT is an aggressive shift from CLL to lymphoma, associated with metabolic changes and aberrations in the MYC transcriptional network. Genomic analysis of paired CLL and RT cases revealed recurrent mutations or deletions in the MYC suppressor Max-gene-associated (MGA) increase from 5% (CLL) to 30% (RT) cases, highlighting its critical yet unexplored role as a driver event. We recently developed a murine RT model using B cell-restricted in vitro editing of LSK progenitors from Cd19Cre-Cas9-del(13q)-Sf3b1 ( Sf3b1-K700E) with Mga-targeting sgRNAs. This murine model demonstrated upregulation of Mga targets Myc and Nme1 (nucleoside diphosphate kinase), a distinct OXPHOS-based transcription signature, increased oxygen consumption rate, elevated ROS levels, suggesting a significant link between the Mga-Myc-Nme1 axis and OXPHOS dysregulation in RT. Given the crucial role of metabolic reprogramming in cancer, we seek to understand the molecular mechanisms of metabolites driving OXPHOS and accelerating CLL transformation. Unraveling these processes could revolutionize therapeutic approaches for managing RT in clinical settings.
To identify metabolites driving dysregulated OXPHOS in RT, we first conducted paired RNA-seq and unlabeled metabolomic profiling on normal and RT mouse splenic B cells (n=3/group). To translate our discovery from the murine RT model to human disease, we performed metabolomic profiling using MEC1 and Nalm6 cell lines with MGA deletion. Our metabolomic analysis of murine RT cells revealed 44 upregulated metabolites, including TCA cycle substrates (glutamine fold change (FC)=9.6, p=0.001;asparagine FC= 4.0, p=0.006), fatty-acid intermediates (glycerol-3P FC=11.7, p=0.003; carnitine FC=4.3, p=0.02; palmitate FC= 4.1, p= 0.08), and glycolysis intermediates (3-phosphoglycerate FC= 4.0, p=0.03; UDP-glucose FC=12.7, p=0.02; glycine FC=4.7, p=0.03). Moreover, integrated metabolite analysis (MetaboAnalyst v5.0) identified 63 common upregulated metabolites in MGA KO (knockout) human cell lines and murine RT, enriching glycerolipid, and fatty acid oxidation, associated pathways, indicating a potential role for MGA deletion in driving dysregulated fatty acid metabolism.
Pathway enrichment analysis using integrated RNA-seq and metabolite analyses showed that the glycerolipid pathway was one of the most enriched pathways among mouse RT and B-cell lines (Nalm6 and MEC1) with MGA KO. Additionally, two key fatty-acid enzymes, fatty acid synthetase (FASN) and phosphoglycerate phosphatase (PGP), displayed significant mRNA and protein changes, as validated by qPCR and immunoblotting. Furthermore, elevated lipid accumulation and increased free glycerol were confirmed based on lipi-green flow cytometry and glycerol-bioluminescent assays, suggesting an important role of glycerolipid metabolism in MGA KO-associated OXPHOS.
PGP, a glycerol-3-phosphatase, regulates glycerol-3-P levels, a key intermediate in glucose, lipid, and energy metabolism. Overexpression of MYC and NME1 increases PGP levels in B-cell lines (MEC1, Nalm6, and Jeko), confirming PGP as a direct target of the MGA-MYC-NME1 axis. To determine the role of PGP in MGA KO (knock-out) cells, we used CRISPR/Cas9 to KO PGP in MEC1, Nalm6, and JeKo cell lines. KO of PGP decreased cell growth and reduced OXPHOS compared to controls based on CCK8 absorbance and Seahorse Mitostress assays respectively. Moreover, PGP KO downregulated the mTOR (p4E-BP1) pathway, emphasizing the significance of dysregulated glycerol and fatty acid metabolism in CLL to RT-driven OXPHOS.
Our study reveals key metabolites driving RT and highlights the crucial role of the MGA-MYC-NME1-PGP axis in regulating OXPHOS in Mga KO RT cells. Our results thus suggest targeting glycerolipid metabolism and related pathways may offer effective strategies to conquer RT
Disclosures
Rosen:Exicure: Current holder of stock options in a privately-held company; Pepromene Bio, Inc: Current holder of stock options in a privately-held company; Pepromene Bio, Inc: Membership on an entity's Board of Directors or advisory committees; NeoGenomics: Membership on an entity's Board of Directors or advisory committees; Verastem, Inc: Consultancy; Trillium Therapeutics, Inc: Consultancy; PharmaGene, LLC: Consultancy; Apobiologix/Apotex Inc: Consultancy; Exicure: Consultancy; Pheromone Bio, Inc: Consultancy; January Biotech: Current holder of stock options in a privately-held company; Trillium Therapeutics: Current holder of stock options in a privately-held company; SLAM BIOTHERAPEUTICS, INC: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees. Danilov:GenMab: Consultancy, Research Funding; Lilly Oncology: Consultancy, Research Funding; Nurix: Consultancy, Research Funding; Bayer: Research Funding; Abbvie: Consultancy, Research Funding; Genentech: Consultancy; Merck: Consultancy; Cyclacel: Research Funding; Janssen: Consultancy; Astra Zeneca: Consultancy, Research Funding; Bristol Meyers Squibb: Consultancy, Research Funding; Beigene: Consultancy, Research Funding; MEI: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal